System Integrating BiopharmaceuticalSolution In One Platform
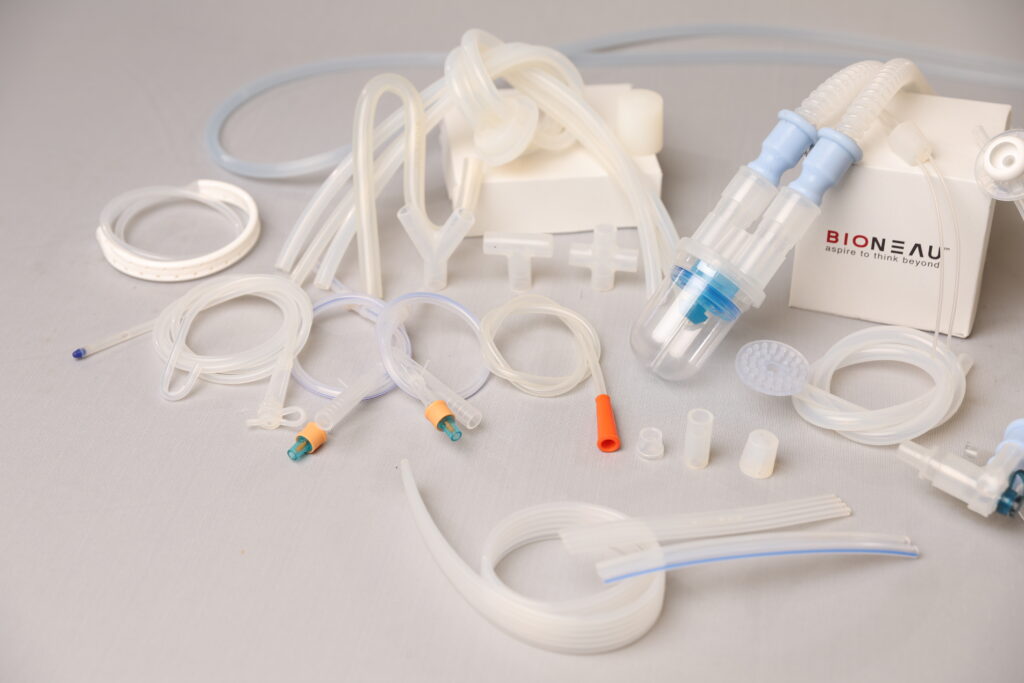
Single-use tubing and integrated assemblies are pre-sterilized, ready-to-use systems designed for fluid transfer in bioprocessing applications. Manufactured and packaged in ISO Class 5–7 cleanrooms, these assemblies ensure sterility and reduce contamination risk. Each assembly is custom-built using pharmaceutical-grade tubing, connectors, filters, and clamps, then sterilized—typically by gamma irradiation.
They are widely used in buffer/media preparation, TFF, chromatography, bioreactor harvest, sampling, and sterile filling. By eliminating the need for cleaning and validation, these systems accelerate changeovers and enhance process safety and efficiency.
Fully customizable in tubing size, layout, and components, they comply with global standards such as USP, ISO 10993, and FDA 21 CFR, making them ideal for use in regulated biopharmaceutical environments.

